1. INTRODUCTION
Zeolites are widely used in catalytic processes due to their acidity, stability and microporous channels [1] [2]. One of their main applications is in the fluid catalytic cracking process [3] in the petroleum refining industry in which the heavy hydrocarbons (chains with more than 20 carbon atoms) are reduced to lighter compounds, in the range of gasoline and diesel [4] [5]. However, heavy hydrocarbons have diffusional transport restrictions into the porous of zeolites, since the molecular size of the majority of these hydrocarbons is higher than the zeolite channels size [6]. The low diffusion of the molecules into the catalyst is responsible of the production of undesired compounds like coke, a carbonaceous macromolecule that deactivates the catalyst [7] [8] [9].
One of the solutions to this problem is the generation of mesoporous channels in the zeolite [1] [10], using post-synthesis treatments as dealumination (using acid treatments [11] or steaming [12]) and desilication [13] [14] (using alkaline treatments), treatments in which atoms of aluminum or silicon are leached from the framework, respectively [15]. In the case of the dealumination process, some acids as nitric [16], citric [17] and oxalic acid [11] have been tested. While mineral acids are stronger and dissociate the zeolites, the best crystalline, textural and catalytic properties have been obtained with lactic [18] and citric [19] acids, due to their bifunctional nature: leaching and chelating nature with Al [20].
The desilication process is mainly carried out with sodium [13] [21] or ammonia hydroxide [22], at atmospheric pressure or under hydrothermal conditions at autogenous pressure, with or without the presence of directing agents (SDA) [23] that protect the structure and avoid the collapse of the framework during the treatments [24] [25]. Then, among the parameters that can affect the synthesis are the amount of SDA, temperature and the hydrothermal treatment time [4] [26].
Sodium hydroxide is stronger than ammonia hydroxide; furthermore, NaOH dissociates immediately in the aqueous media, while NH4OH forms an equilibrium and its dissociation occurs slowly, giving a controlled desilication of zeolite [27]. Furthermore, if NaOH is used for the treatments, an ion exchange step is necessary later to reactivate the catalyst [28]. Then, the use of ammonia hydroxide in the production of hierarchical zeolites using desilication process, is preferred instead of NaOH.
The Si extraction is efficient in zeolites with a Si/Al ratio higher than 25 [28]; otherwise, acid and alkaline treatments are necessary to obtain mesoporous in zeolites [29]. Verboekend et al. [30] synthesized hierarchical zeolites from a zeolite Y with a Si/Al ratio of 2.6. The authors included acid treatment with H4EDTA, alkaline procedure with NaOH and washing with Na2H2EDTA to clean the surface of the material from debris generated in the other steps and ion exchange step to remove sodium cations.
On the other hand, Javier García et al. [23] synthesized a hierarchical zeolite Y using NH4OH and cetyltrimethylammonium bromide (CTAB) in a hydrothermal treatment for the desilication procedure, starting from a zeolite with Si/Al = 15 (0.84 g CTAB/g NH4OH, 10 h of hydrothermal treatment, 150°C). High mesopore surface area was obtained and crystallinity of the material was maintained with this procedure. Although the mesoporous surface area increased, the effect of the treatment parameters on the crystalline and textural properties was not specified. In this research, the effect of the amount of CTAB and the hydrothermal treatment time on the desilication process of a zeolite with a Si/Al = 15 (CBV720, from Zeolyst), based on the synthesis reported by Javier García et al. [23] is analyzed.
In a complementary study, a zeolite with a Si/Al lower than 15 was dealuminated, desilicated and acid washed, and the obtained material was compared with the material reported by D. Verboekend, et al. [30]. The desilication process was carried out with the amount of CTAB and hydrothermal treatment time selected from results obtained with CBV720 material. H4EDTA and Na2H2EDTA were used for the dealumination and acid washing steps, respectively. The objective was to analyze the effect of this desilication step with NH4OH assisted by CTAB in a zeolite with a Si/Al < 15 avoiding the use of NaOH.
2. METHODOLOGY
2.1. Reagents
The parent zeolites used in this research were zeolite Y with Si/Al = 2.6 (CBV500) and zeolite Y with a Si/Al = 15 (CBV720), both purchased to Zeolyst International. Ammonia hydroxide solution (NH4OH, 28-30%) and ethylenediaminetetraacetic acid (H4EDTA, 99.8%) from Merck, ethylenediaminetetraacetic disodic acid (Na2H2EDTA, 99%) from Sigma-Aldrich, and cetyltrimethylammonium bromide (CTAB, >97%) from J.T. Baker. All the reagents were used as purchased.
2.2. Synthesis of materials
2.2.1. Effect of the amount of surfactant in the desilication process
For the analysis of the influence of the amount of surfactant on the desilication process, the synthesis proposed by Javier García et al. [23] was followed. Different amounts of surfactant were mixed with 64 mL of ammonia hydroxide solution 0.37 M in a mass ratio of 0 to 1.25 of CTAB/NH4OH, then 1 g of the parent zeolite Y (CBV720) was dispersed in the solution and mixed at room temperature for 20 minutes. Then, the dispersion was autoclaved for the hydrothermal treatment 10 h at 150°C. After the treatment, the samples were washed until achieving the pH of deionized water, filtered and dried at 80°C for 14 h. Finally, the calcination of samples was made at 550°C with a temperature rate of 5°C/min, 2 h under nitrogen atmosphere and then 2 h with dry air.
The samples were named Z720-X, were X corresponds to CTBA/NH4OH mass ratio (0, 25, 35, 75, 100 and 125) in the aqueous media used in the desilication step. The amounts of CTAB used in the procedure was higher than the critic micellar concentration (CMC = 9.2x10-4 M) of CTAB [31] [32], to guarantee the formation of micelles in the media.
2.2.2. Effect of the hydrothermal treatment time on the desilication process
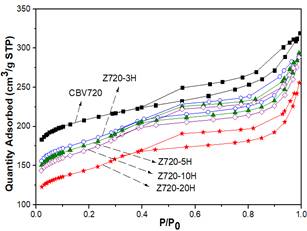
To analyze the influence of the hydrothermal treatment time on the desilication process, the synthesis reported by Javier García et al. [23] was followed. A solution was prepared with 0.2075 g of CTAB and 64 mL of ammonia hydroxide solution 0.37 M, then 1 g of commercial zeolite Y (CBV720) was added and the mixing continued for 20 minutes at room temperature. Then, the dispersion was autoclaved for the hydrothermal treatment for 3, 5, 10 and 20 h at 150°C.
After the treatment, the samples were washed until pH of deionized water was achieved, filtered and dried at 80°C for 14 h. Finally, the calcination of the samples was made at 550°C with a temperature rate of 5°C/min, 2 h with nitrogen and then 2 h with dry air. The samples were named as Z720-YH, with Y the hydrothermal treatment time.
2.2.3. Effect of the desilication agent type on the hierarchical zeolite synthesis
For the modification of the zeolite Y with Si/Al = 2.6, the commercial zeolite CBV500 was dealuminated and acid washed following the procedure reported by D. Veboekend et al. [30] and desilicated according to Javier García et al. [23], with the amount of CTAB and hydrothermal treatment time found with the samples Z720-X and Z720-YH. First, 6.7 g of zeolite Y was added to H4EDTA 0.15 M at 100°C for 6 h. The alkaline treatment was carried out using a ratio of 1 g zeolite to 64 mL of NH4OH 0.37 M, CTAB was added in this step in a ratio of 0.25 g CTAB/g NH4OH; then, the suspension was stirred for 20 min at room temperature. After that, the suspension was autoclaved for hydrothermal treatment at 150°C for 10 h. The solid was filtered and washed until pH of deionized water. Finally, the acid washing of the zeolite was carried out at 100°C using Na2H2EDTA 0.11 M for 6 h in a ratio of 6.7 g of zeolite to 100 mL of solution.
Between all the steps, the material was washed and dried at 65°C in the case of acid treatments and at 80°C for the alkaline treatment. The powder was calcined at 550°C for 5 h twice with a heating rate of 5°C/min under static air. The modified sample was named as Z500-VG.
2.3. Characterization of samples
X-Ray diffraction (XRD) analysis was carried out using a Panalytical Empyrean X-Ray diffractometer with a Co Kα radiation (λ =0.154 nm) generated at 40 kV and 40 mA. The 2θ was scanned from 5 to 55°.
The relative crystallinity (%RC) was calculated for all the samples following the ASTM D3906-03 [33], using Equation (1) and the PANalytical X’Pert Highscore Plus software. The peaks selected for the analysis were at 2θ = 22°, 24°, 28° and 32° that correspond to the crystallographic planes of (544), (440), (533) and (642), respectively.
|
|
(1) |
With the N2 adsorption/desorption isotherms the textural properties of the samples were obtained, using a Micromeritics ASAP 2020 Plus equipment. With the t-plot method the micropore surface area and volume were calculated; the mesopore surface area and volume were obtained with the BJH correlation and the surface area with the BET method, using the adsorption branch of the isotherms. With this information, the hierarchical factor (HF) was calculated; the HF brings information about the relation between the destruction of microporous and the generation of mesoporous. Zheng et al. [34] proposed a way to calculate that factor including textural properties obtained from the nitrogen physisorption analysis, Equation (2).
|
|
(2) |
Where micropore volume (Vmicro), specific surface area of the mesopores (Smeso), mesopore volume (Vmeso) and the BET surface area (SBET) are related.
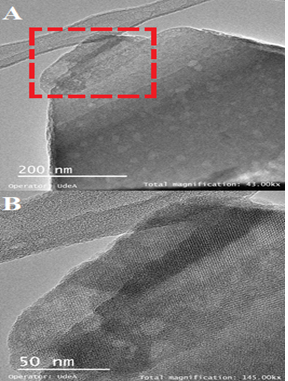
Transmission electron microscopy (TEM) was carried out in a Tecnai F20 Super Twin TMP equipment using an accelerating voltage of 200 kV. The samples were added to a mixture of water and ethanol and dispersed for 10 minutes using an ultrasonic equipment; a drop of the suspension was placed in the Cu grill.
3. RESULTS AND ANALYSIS
3.1. Effect of the amount of SDA on the desilication process
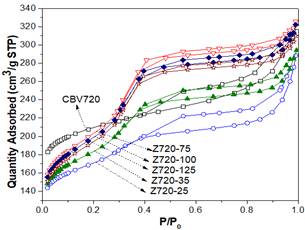
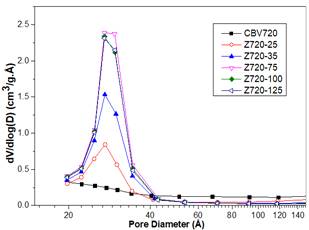
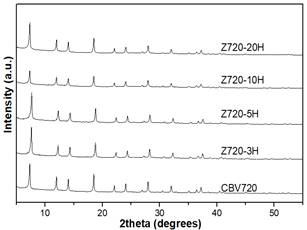
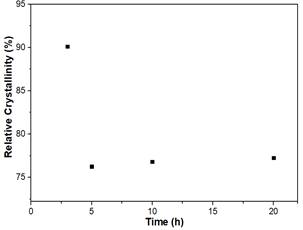
In this step, different amounts of CTAB were added to the alkaline solution, previous to the desilication of the zeolite Y CBV720. Fig 1 shows XRD pattern of Z720-X samples. When a surfactant was not used in the synthesis (Z720-0), the crystalline phase of the zeolite was completely lost. However, when the surfactant was used in the desilication process (samples Z720-25 to Z720-125), the typical peaks of zeolite Y were obtained; the lower intensity of the peaks of the desilicated samples compared with the parent zeolite (CBV720), it is related with the desilication of the sample, which partly destroys the crystalline phase of the samples.
Fig 2 shows that there is an inverse relation between the CTAB/NH4OH mass ratio and the %RC; an increase in the amount of CTAB reduces the %RC of the zeolite. This could be owing to the increase of the amount of micelles formed in the aqueous media. Consequently, an interaction of CTAB exists with the framework of the zeolite that is partially dissolved at an alkaline media [17] [23].
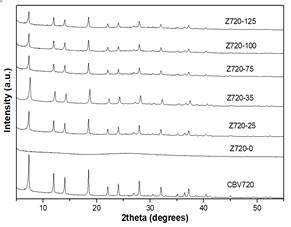
Fig 1. XRD of Z720-X samples and the parent zeolite (CBV720).
The interaction between the micelles and the framework is good with low amount of surfactant, but when it increases, the recrystallization of the framework is altered and the correct rearrangement of the atoms is disturbed, generating amorphicity in the material. When the CTAB/NH4OH mass ratio increases from 0.25 to 1.25, a decrease of 20% is observed in the %RC.
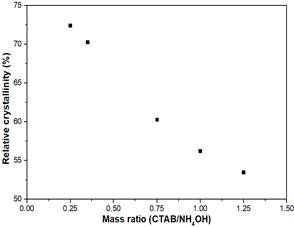
Fig 2. Effect of CTBA/NH4OH mass ratio on relative crystallinity.